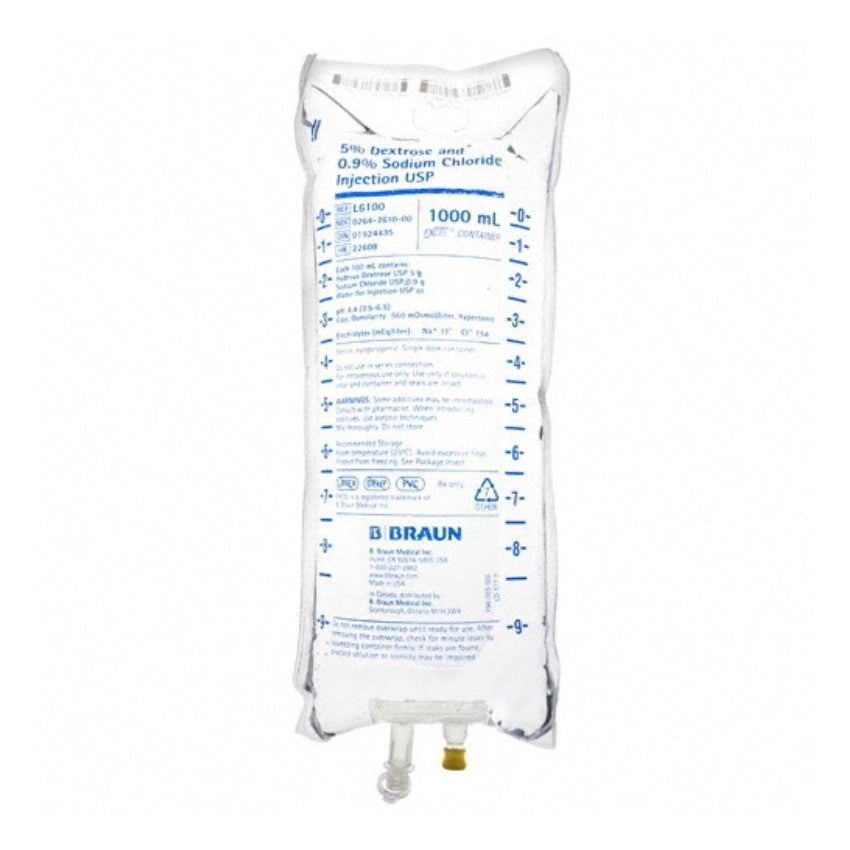
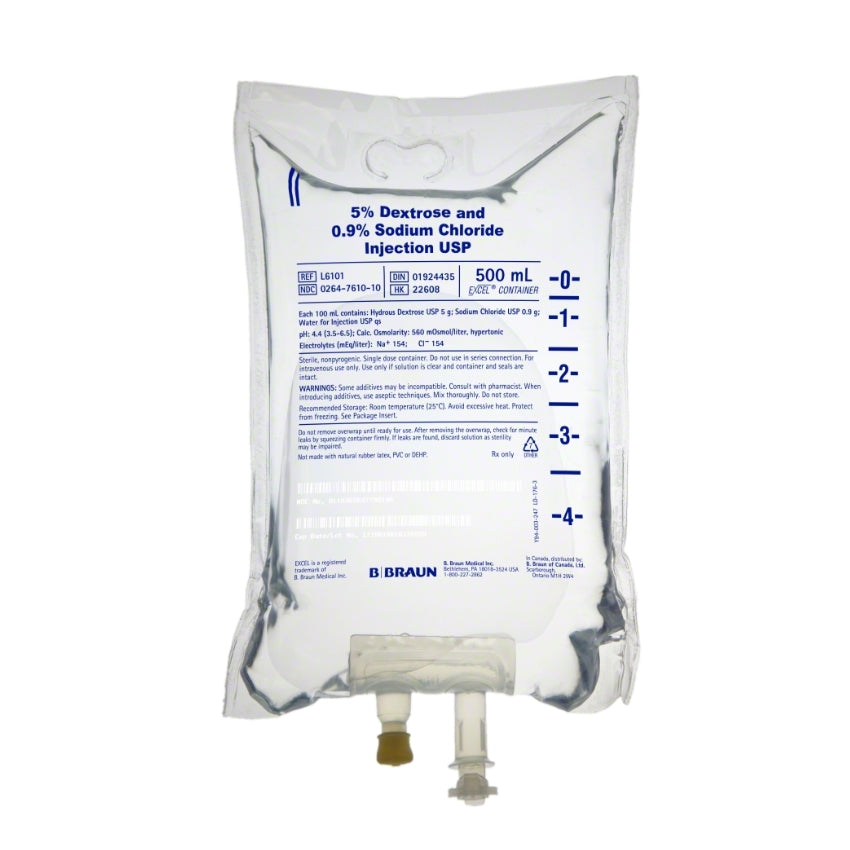
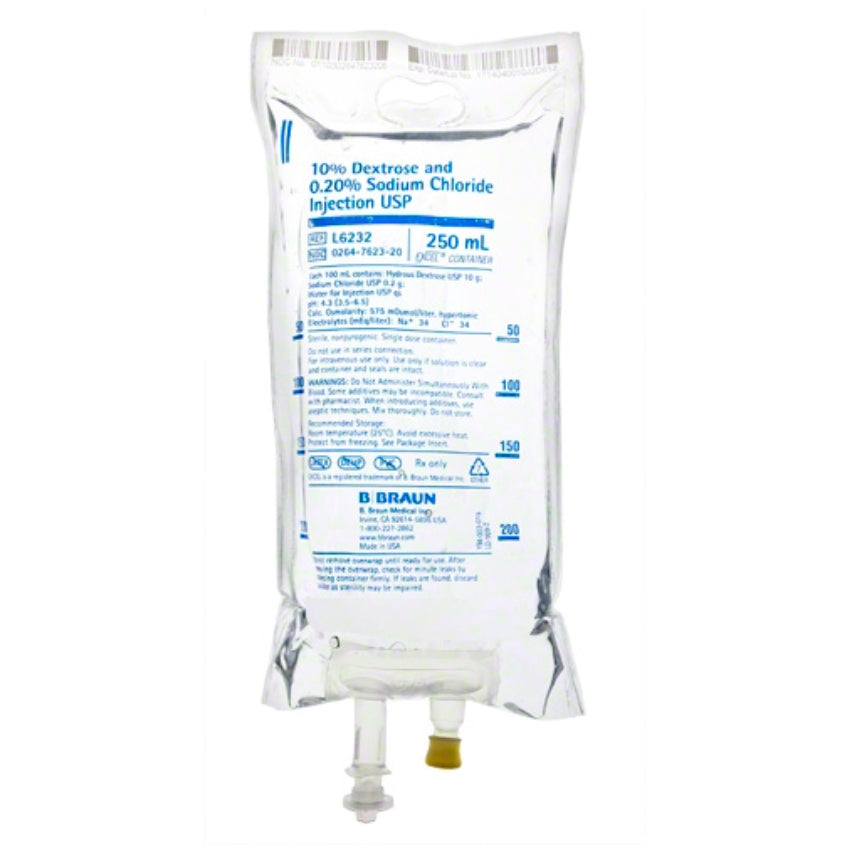
Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes, calories and water for hydration.
Features
- Excel containers feature biologically inert, non-toxic plastic construction
- Unique PVC-Free and DEHP-Free design provides for a safe, accurate means of administering IV medication
Options available
BBNL6100 – EXCEL IV Container | 5% – 0.9% Strength | 1000 mL Volume [12/case]
BBNL6101 – EXCEL IV Container | 5% – 0.9% Strength | 500 mL Volume [24/case]
BBNL6220 – EXCEL IV Container | 10% – 0.45% Strength | 1000 mL Volume [12/case]
BBNL6232 – EXCEL IV Container | 10% – 0.45% Strength | 250 mL Volume [24/case]