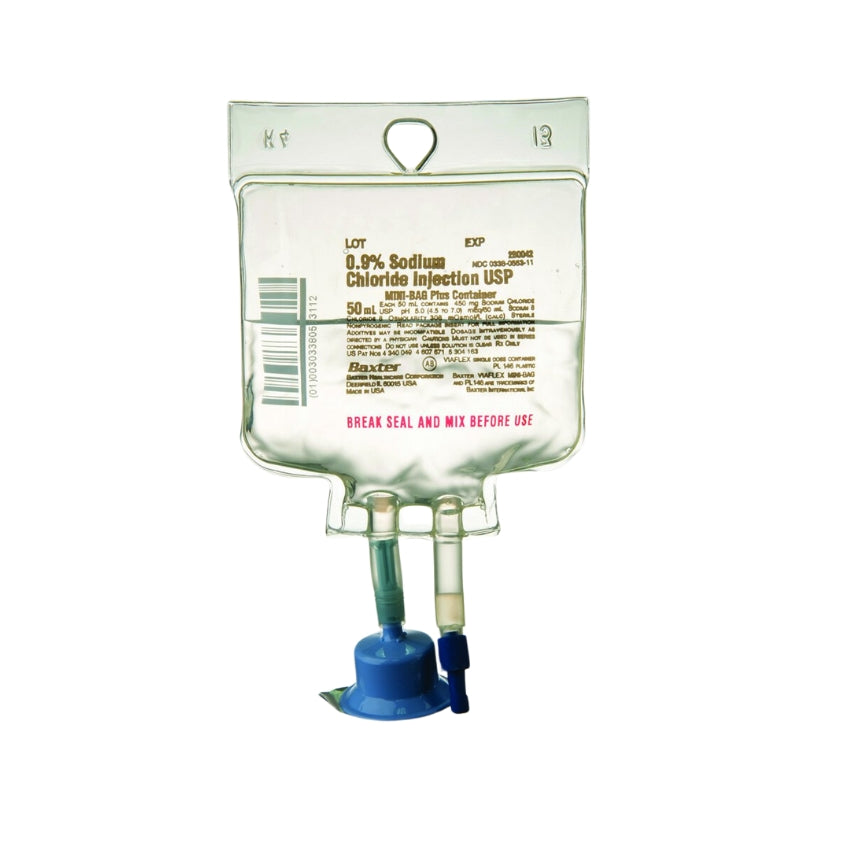
0.9% Sodium Chloride Injection, USP in the MINI-BAG Plus Container is a sterile, nonpyrogenic solution for intravenous administration after admixture with a single dose powdered or liquid (up to 10 mL) drug vial. It contains no antimicrobial agents. The nominal pH is 5.0 (4.5 to 7.0). Each 100 mL contains 900 mg of Sodium Chloride, USP (NaCl). The osmolarity is 308 mOsmol/L (calculated). It contains 154 mEq/L sodium and 154 mEq/L chloride.
Features
- The MiniBag Plus product mechanically prohibits the transfer of contaminants into and out of the system during and after docking, minimizing environmental and personal exposure.
-
Sodium Chloride Injection, USP has value as a source of water and electrolytes. It is capable of inducing diuresis depending on the clinical condition of the patient.
Sold by
0.9% Sodium Chloride Injection, USP, Mini Bag Plus Container. Quad Pack [80/case]